An overview of the regulatory approaches to genome editing of plants in different countries across the world has just been published.
With the European Commission set to present a proposal on a new regulation for genome-edited crops and plants, expected in Q2 2023, the need to critically assess what such regulation might look like is more important than ever. The current food crisis – which has brought back “food sovereignty” to the centre of the political debate – on the one side, and the impact of climate change in terms of droughts and floods on the other, make it clear that there is a need for different approaches to agriculture. It is necessary to look into the possible policy options for a future-proof regulation of genome-edited plants and crops and how this issue is being addressed in the various jurisdictions.
Establishing such a benchmark was the main purpose of the event “Genome Editing Beyond the EU: A Global Conversation”, organised by Re-Imagine Europa together with knowledge partners EU-SAGE and ALLEA. Dr Steffi Friedrichs (Special Advisor on RIE’s Planet Task Force, Founder & Director of AcumenIST SRL), Dr Karinne Ludlow (Monash University) and Dr Peter Kearns (RIE’s Director for Sustainable Food Systems and Innovation, Founder & Director of SwiftEST SARL) have contributed a chapter to the recently published book “Applications of Genome Modulation and Editing” (book series: Methods in Molecular Biology, MIMB, volume 2495).
In their chapter, the three authors analyse and discuss 25 countries and regions that have issued results or have ongoing investigations and discussions about genome editing governance in their jurisdictions. As part of their analysis of the genome editing policies, the authors highlight some countries’ decision-making processes, such as that of Argentina (Whelan & Lema, 2015), joint statements (such as that of Argentina, Chile, Brazil, Paraguay and Uruguay) [1], and proposed risk assessment processes such as that of India (at the time of writing, 2021)[2].
The book chapter provides a background to the development of genome editing techniques against the backdrop of other ‘new (plant) breeding techniques’ (N(P)BTs) and ‘genome modification’ (GM). The chapter goes on to clarify the history and meanings of regulatorily relevant definitions, such as the term living modified organisms (LMOs) of the Cartagena Protocol on Biosafety (CPB) [3]. It also provides technical details and distinctions between the four main known genome editors (i.e. meganucleases, zinc-finger nucleases, TALENs, and CRISPR/Cas) and the three main types of genome editing: SDN1, SDN2, SDN3 (SDN = site-directed nuclease).
Faced with the challenge of concluding the chapter with recommendations about the most appropriate policy on genome editing, Dr Friedrichs, Dr Ludlow, and Dr Kearns use the universal principles of all good regulation (i.e. (i) proportionality, (ii) non-discrimination, (iii) predictability, and (iv) enforceability) as benchmarks to highlight those regulatory actions that are particularly conducive to supporting the principles. In this context, the authors discuss the case of Argentina, where the exemption of SDN1- and SDN2-edited plants from GMO regulations in 2015 triggered a significant increase in applications for GMO-deregulation applications by both small- and medium-sized enterprises (SMEs), local companies and public research laboratories [4].
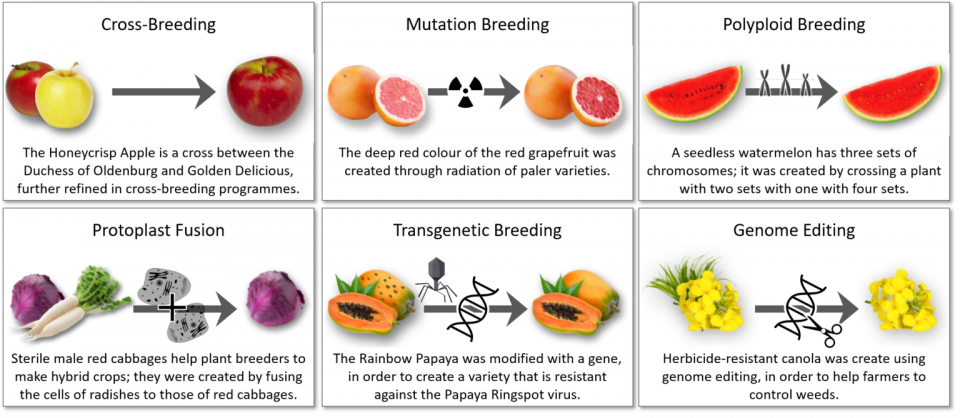
Figure: Illustration of plant breeding techniques (incl. genome editing) (source Biology Fortified [5] and [6]).
[1] Website: Cartagena Protocol on Biosafety (accessed: 10th March 2021)
[2] Genetic Editing Techniques, Southern Agricultural Council (CAS) XXXV Regular Meeting, 20th September 2018. (accessed: 1st March 2021)
[3] Government of India (January 2020): Draft Document on Genome Edited Organisms: Regulatory Framework and Guidelines for Risk Assessment(accessed: 7th March 2021)
[4] Whelan, A., Gutti, P., & Lema, M. (4 de 2020). Gene Editing Regulation and Innovation Economics. Frontiers in Bioengineering and Biotechnology, 8.
[5] Website: Biology Fortified (accessed: 29th April 2021)
[6] Ahmad, S., Wei, X., Sheng, Z., Hu, P., & Tang, S. (7 de 2018). CRISPR/Cas9 for development of disease resistance in plants: Recent progress, limitations and future prospects. Briefings in Functional Genomics, 19(1), 26-39.